SourceWeb® Antigen Self Test
Officially recognized in the EU as a lay test (with CE seal).
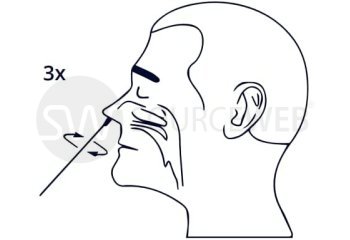
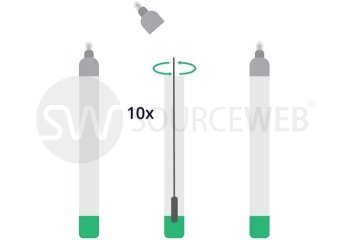
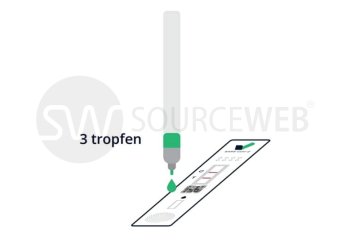
This rapid, single-use test is used for the determination of coronavirus antigen. The sample is taken from a human nasal swab. The Corona Rapid Test is used as a rapid test for suspected coronavirus cases. This test can also be used for reconfirmation for nucleic acid detection in discharged patients. This test has CE approval for lay use (self-testing) for the detection of SARS-CoV-2 and is also listed with the BfArM, as well as evaluated by the Paul Ehrlich Institute.
Special features:
This rapid, single-use test is used for the determination of coronavirus antigen. The sample is taken from a human nasal swab. The Corona Rapid Test is used as a rapid test for suspected coronavirus cases. This test can also be used for reconfirmation for nucleic acid detection in discharged patients. This test has CE approval for lay use (self-testing) for the detection of SARS-CoV-2 and is also listed with the BfArM, as well as evaluated by the Paul Ehrlich Institute.
Special features:
- Rapid result - 15-20 minutes
- Easy to use even by laymen - All in one kit
- Hygienically individually packaged test kits
- High accuracy and variant tested (including Omicron).
- Test with CE certification as layman test
Scope of delivery:
- Coronavirus antigen test cassette
- Sample extraction buffer
- Sterile swab
- Disposable pressure bag for disposal
- Instructions for use
Manufacturer: SourceWeb Medical Inc.
Instrument: Can be performed without additional equipment
Format: Anterior nasal test
Storage temperature: 4 - 30°C
Selection time: 15 minutes
Specificity: 99,76 %
Sensitivity: 97,10%
CE Certification: CE 0865
Packaging: Pack of 10 pieces
Packaging and design may differ from the picture