SafeCare Antigen Self Test
The SafeCare COVID-19 Nasopharyngeal Antigen Rapid Test is designed to provide high-quality detection of nucleocapsid protein antigens. To do this, a qualified swab is taken from the nasopharynx or anterior nasal region as part of a professional testing strategy. The antigen rapid test has been evaluated according to the Paul Ehrlich Institute and is included on the BfArM list for SARS-CoV-2 tests. It may only be performed by medically qualified personnel. Testing by trained personnel is also possible at the PoC.
Manufacturer SafeCare brings the COVID-19 Nasopharyngeal Antigen Rapid Test, a reliable and simple option to implement effective testing for the novel coronavirus. Since mass testing is also possible, a sustainable testing strategy can be implementedforpublic facilities or commercially used areas as well as schools and companies in orderto quickly locate people infected with SARS-CoV-2 and thus protect all persons concerned.
A test result is available within 10 to 15. Thus, those tested have rapid certainty, with the relative sensitivity of the SafeCare - COVID-19 Nasopharyngeal Antigen Rapid Test The relative specificity is 96.97% and the relative specificity is 100%.
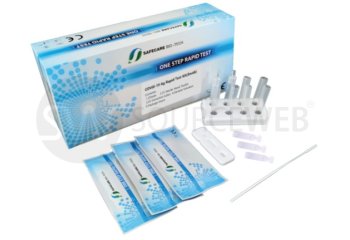
Specimen Material: nasopharyngeal swabs.
Test result: in 10 to 15 minutes
Very high sensitivity: 96.97%
Very high specificity: 100.00%
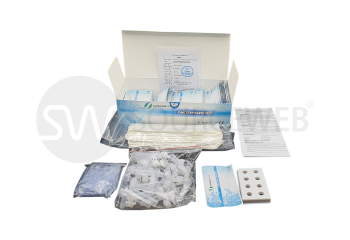
Contents : 25 tests incl. consumables
Handling: Easy handling without additional analysis system
Test location: Test possibility where no laboratory test is available (PoC)
Use: Testintended for professional use
Reimbursable: BfArM-listed (AT199/20) and therefore reimbursable.
The indicated prices are unit prices per 1 test